How ERP Systems Simplify Regulatory Compliance

Struggling with regulatory compliance? ERP systems can help.
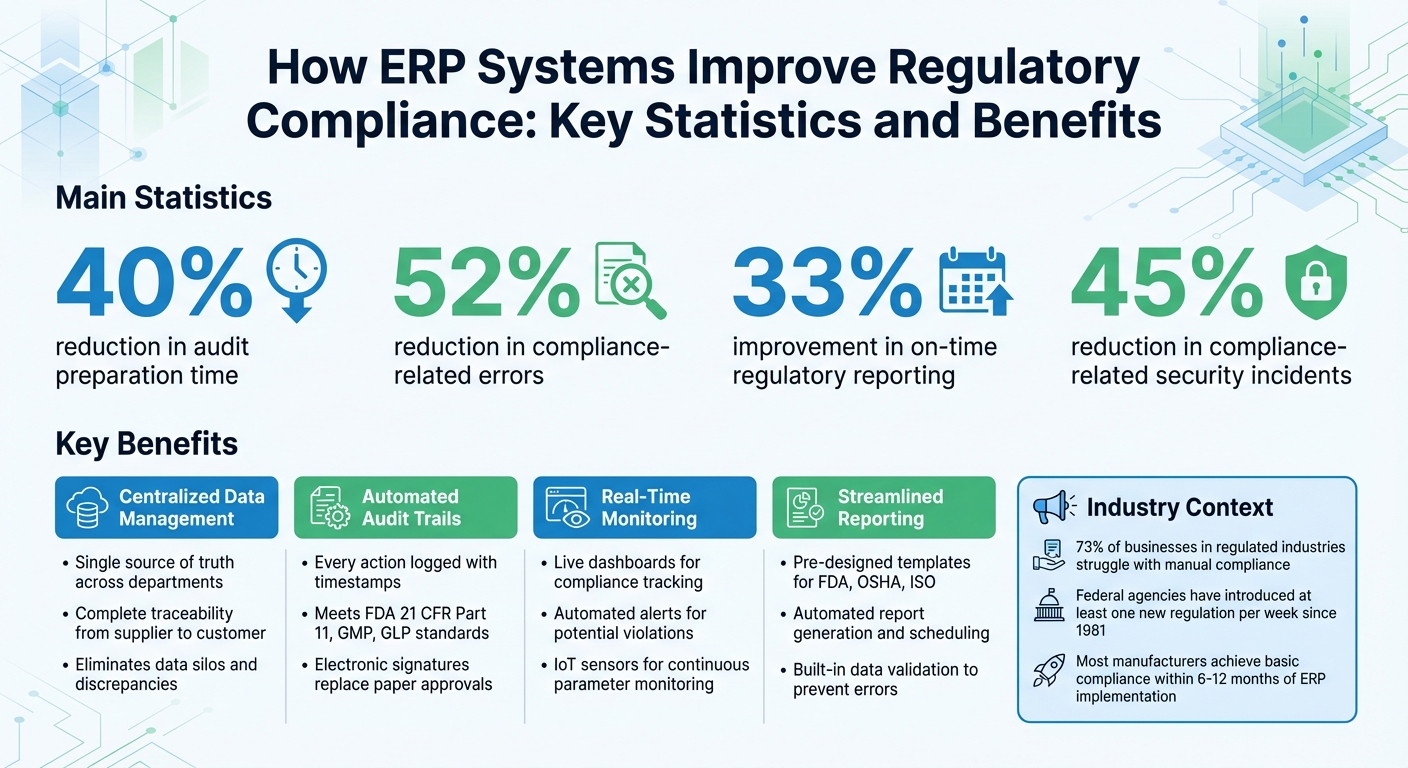
ERP (Enterprise Resource Planning) systems simplify compliance by centralizing data, automating documentation, and enabling real-time monitoring. This eliminates manual errors, reduces audit preparation time by 40%, and cuts compliance-related mistakes by 52%. For manufacturers, this means less stress, faster audits, and fewer penalties.
Key benefits include:
- Centralized data: A single source of truth ensures consistency across departments.
- Automated audit trails: Every action is logged with timestamps for full transparency.
- Real-time monitoring: Alerts flag potential violations before they escalate.
- Streamlined reporting: Pre-designed templates save time and improve accuracy.
ERP systems not only simplify compliance but also support business growth by reducing risks and improving operational efficiency.
How ERP Systems Improve Regulatory Compliance: Key Statistics and Benefits
Sage X3 - Discrete Manufacturing - Regulatory Compliance
Centralized Data Management for Compliance
Efficient data management forms the backbone of modern ERP systems, playing a crucial role in proactive compliance efforts. One of the standout features of an ERP system is its ability to bring together data from all areas of operation into a single, unified database. This ensures everyone works with consistent, real-time information. For instance, the arrival of raw materials can be logged and tracked automatically through production and shipping. This complete traceability allows companies to track a finished product back to its original supplier or forward to the receiving customer - an essential capability for meeting regulatory demands or managing product recalls.
Automating compliance documentation reduces errors by 52% compared to manual processes [5] and improves on-time reporting by 33% [5]. These gains stem from streamlined data entry, where information is input once and shared across the system, eliminating duplication. This centralized approach not only simplifies compliance management but also sets the stage for smoother audit processes.
Improved Data Access for Auditors and Regulators
Centralized data significantly simplifies audit preparation. Instead of spending days - or even weeks - gathering documents from various departments, companies can assemble audit packages in just a few hours. Auditors can quickly move from high-level dashboard summaries to detailed source documents, such as vendor invoices or quality control reports, with just a few clicks. This streamlined access reduces audit preparation time by up to 40% for ERP users [5].
Role-based permissions ensure that only authorized individuals can access sensitive compliance-related information. This setup not only keeps regulators informed with up-to-date, accurate data but also eliminates the last-minute scramble to compile documentation. While improved access speeds up audits, breaking down departmental silos ensures data remains consistent throughout the compliance process.
Eliminating Data Silos and Discrepancies
Data silos often lead to compliance headaches. For example, mismatches between production records and inventory counts or disconnected quality test results and shipping logs can create discrepancies. These inconsistencies complicate audits and slow down responses to potential safety issues. Centralized ERP systems solve these problems by ensuring all departments rely on the same accurate, unified dataset. If quality control flags a defective batch, the system can automatically halt its progression. Similarly, finance teams can reconcile material costs using production data already logged in the system. This level of consistency is critical for meeting regulations like the Sarbanes-Oxley Act (SOX), which requires public companies to maintain integrated internal controls.
Security is another major advantage. ERP systems with robust access controls can cut compliance-related security incidents by over 45% [5]. By implementing granular, role-based permissions, employees only access the data necessary for their specific roles, supporting compliance with regulations such as HIPAA and GDPR. Additionally, enforcing segregation of duties - such as ensuring the person entering data isn’t the same person approving it - helps maintain audit trail integrity and reduces the risk of fraud.
Automated Audit Trails and Compliance Documentation
Automated audit trails, built on centralized data management, play a critical role in meeting regulatory requirements by logging every key system interaction. Every ERP action - whether it’s a login, data entry, or approval - is automatically recorded with essential details like who performed the action, what was done, and when it occurred. This creates a transparent, permanent record that aligns with regulations such as FDA 21 CFR Part 11, Good Manufacturing Practice (GMP), and Good Laboratory Practice (GLP) [2][1].
"Always-on audit trails let you track changes to transactions and data, including what was changed, who changed it and when."
– Lisa Schwarz, Senior Director of Global Product Marketing, NetSuite [1]
These digital logs eliminate the risks tied to paper-based systems, such as illegible handwriting, misplaced documents, or unauthorized modifications. ERP systems also provide version control for critical documents like formulas and standard operating procedures (SOPs), ensuring teams always work with the latest approved version [5]. Additionally, electronic signatures with timestamps replace traditional paper approvals, adding another layer of security and accountability [3][6]. This detailed documentation is invaluable during audits, allowing regulators to verify that every manufacturing step adhered to approved protocols. The following sections explore how timestamped logs and digital batch records enhance traceability and transparency.
Timestamped Audit Trails for Transparency
Timestamped records offer the accountability regulators require. For instance, if a quality control issue arises with a batch, the audit trail can pinpoint the operator who dispensed materials, the equipment used, and the exact timing of each production step. This level of detail is essential for compliance with Title 21 CFR Part 211.188.
Electronic Batch Records and Documentation
Electronic batch manufacturing records (EBMR) replace traditional paper logbooks with digital documentation, capturing every stage of the production process. ERP systems create two key types of records: the Master Batch Record (MBR) and the Control Batch Record (CBR).
| Feature | Master Batch Record (MBR) | Control Batch Record (CBR) |
|---|---|---|
| Purpose | Template/Instructions for manufacturing | Record of actual production events |
| Content | Recipes, formulas, SOPs, and material requirements | Actual weights, timestamps, operator IDs, and test results |
| Timing | Created and approved before production starts | Created when the batch starts; updated in real time |
| Regulatory Focus | 21 CFR Part 211.186 | 21 CFR Part 211.188 |
The MBR serves as the approved guide, laying out recipes, formulas, material requirements, and SOPs. Meanwhile, the CBR captures real-time data during production, including material weights, dispensing activities, equipment IDs, and quality test results [6]. This digital "diary" ensures complete traceability, enabling manufacturers to track a finished product back to specific raw material lots or forward to customers in case of a recall [3][1]. When auditors request documentation, companies can produce comprehensive records in hours - far faster than the weeks often needed with manual systems.
Real-Time Monitoring and Compliance Management
Real-time monitoring takes compliance management to the next level by moving from merely reacting to problems to actively preventing risks. Thanks to centralized data management and automated audit trails, ERP systems provide live updates on production activities through customizable dashboards designed for specific roles. For instance, production planners can oversee batch progress in real time, while quality controllers can instantly review test results. This level of visibility allows teams to address potential compliance risks before they turn into full-blown violations [2][4]. It all builds on the solid, unified data foundation that modern ERP systems establish.
Many ERPs now incorporate IoT sensors that continuously track equipment performance and production conditions. If certain parameters - like temperature or contamination levels - exceed regulatory limits, the system sends immediate alerts to administrators. In high-stakes situations, the ERP can even halt workflows automatically until the issue is resolved, ensuring that non-compliant products don’t move forward in the production process [5].
"Automated monitoring systems help prevent compliance issues before they become violations."
– Top10ERP [3]
These systems also flag unauthorized access, missed inspections, or data changes, complete with critical, timestamped details. This functionality is especially important for meeting stringent regulations like FDA 21 CFR Part 11 [1][8]. Let’s dive into how ERP dashboards and automation not only detect but actively prevent compliance violations.
Detecting and Preventing Compliance Violations
ERP dashboards act as a live compliance scorecard, offering a real-time overview across the organization. For example, if an operator skips a required quality check or inputs data that falls outside acceptable parameters, the system immediately alerts the relevant personnel. Alerts can be tailored to match the severity of the issue - minor deviations might notify a shift supervisor, while critical violations could escalate directly to senior management and even pause production lines [5].
Advanced features like pattern recognition further enhance compliance. These systems can identify unusual activities, such as repeated login attempts to restricted areas or unexpected alterations to approved formulations. This proactive approach is crucial, particularly as 73% of businesses in regulated industries report struggling to keep up with evolving compliance standards when relying on manual systems [5]. These capabilities highlight the stark difference between traditional and ERP-driven compliance processes.
Manual Processes vs. ERP Automation
The difference between manual compliance methods and ERP automation becomes evident when comparing their efficiency and ability to manage risks:
| Feature | Manual Processes | ERP-Automated Processes |
|---|---|---|
| Audit Preparation Time | Weeks of preparation [3] | Hours to generate packages [3] |
| Error Risk | High; prone to human error [5][3] | 52% reduction through automation [5] |
| Preparation Efficiency | Reactive; scrambling to gather files [3] | 40% faster preparation [5] |
| Monitoring Approach | Reactive; post-escalation identification [3] | Proactive; early issue flagging [5][3] |
| Data Integrity | Low; redundant entry creates discrepancies [1] | High; single source of truth [5][1] |
Organizations using ERP systems for regulatory reporting have seen a 33% improvement in on-time submissions, while access controls have cut compliance-related security issues by over 45% [5]. By automating compliance monitoring, companies can shift their focus from tedious administrative tasks to addressing strategic risks and improving overall operations.
sbb-itb-e766981
Automated Reporting and Regulatory Submissions
ERP systems streamline the creation of compliance reports for agencies like the FDA, OSHA, and ISO by automating the process. They pull data directly from a centralized database and format it to meet each agency's specific requirements, cutting out the weeks of manual work typically involved.
Accurate and Timely Report Generation
With ERP systems, regulatory submissions are prepared using pre-designed templates specific to each agency. These templates ensure reports meet formatting and data standards. The system consolidates information from various departments - such as production logs, quality tests, employee training records, and financial data - into standardized reports. You can even schedule these reports to be automatically generated on a monthly, quarterly, or annual basis, removing the stress of last-minute deadlines [5].
"ERP systems simplify [reporting] with customizable templates, data export tools, and automated reporting schedules. These features ensure the accuracy and timeliness of reports, whether they are monthly, quarterly, or annual."
– DXoneERP [5]
Organizations using ERP systems have reported a 33% improvement in meeting submission deadlines compared to those relying on manual methods [5]. For instance, in 2025, Noumed Life Sciences, a UK-based pharmaceutical company, adopted SAP Business ByDesign to replace their spreadsheet-based workflows. This shift integrated forecasting, inventory, and production planning, allowing them to achieve real-time visibility and maintain compliance with MHRA standards without the delays caused by manual data handling [7].
This level of automation not only saves time but also significantly reduces the risk of errors in reports. Accurate submissions contribute to stricter quality controls, which in turn help prevent reporting mistakes.
Reducing Errors in Regulatory Submissions
Manual data entry is prone to errors like typos or missing details, which can lead to penalties. ERP systems tackle this issue by incorporating data validation steps that flag inconsistencies before reports are finalized. This built-in quality check reduces submission errors by 52% [5].
Additionally, electronic signatures compliant with FDA 21 CFR Part 11 eliminate the inefficiencies of paper-based approvals while maintaining legal integrity. For example, when a quality manager approves a batch record, the system timestamps the action and locks the data to prevent further changes, creating a secure and unalterable audit trail. Auditors can trace any data point back to its source - whether it’s a vendor invoice, production log, or inspection record - without the need for time-consuming manual searches [5].
Key Benefits of ERP Systems for Regulatory Compliance
ERP systems transform compliance from a challenge into an opportunity for growth.
Reducing Penalties and Ensuring Compliance
Failing to meet regulatory requirements can be costly - both financially and reputationally. Companies risk penalties, business interruptions, lost revenue, and productivity setbacks, all of which can add up to millions of dollars in losses [1]. ERP systems tackle these risks head-on by addressing data inconsistencies, missed deadlines, and gaps in documentation.
By automating key processes, ERP systems minimize errors and make audit preparation far more efficient [5]. This not only saves time but also reduces the chances of costly mistakes that could attract regulatory attention.
"Noncompliance costs thousands to millions... including losses from penalties, business disruption, lost revenue, productivity loss and reputational damage."
– Lisa Schwarz, Senior Director of Global Product Marketing, NetSuite [1]
Take the manufacturing sector as an example: 73% of businesses in regulated industries struggle to keep up with ever-changing compliance standards [5]. In the U.S. alone, federal agencies have introduced at least one new regulation every week since 1981 [1]. ERP systems help businesses keep pace by centralizing data, automating audit trails, and providing real-time monitoring to catch potential issues before they escalate into penalties.
These streamlined compliance processes also enhance a company's ability to adapt quickly to market changes.
Faster Market Entry and Competitive Advantage
By reducing compliance risks and penalties, ERP systems pave the way for quicker regulatory approvals and greater agility in the marketplace. In industries where speed is crucial, these systems simplify the approval process with built-in compliance workflows and automated documentation. Many manufacturers achieve basic compliance within just 6–12 months of implementing an ERP system [3].
Companies using ERP tools for regulatory reporting see a 33% improvement in on-time submissions compared to those relying on manual methods [5]. This reliability allows for faster market entry, eliminating the chaos of last-minute fixes and repeated submissions. Additionally, the ability to generate comprehensive audit reports in hours - rather than weeks - turns audits into opportunities to showcase operational efficiency [3].
Centralized data management ensures consistent operations while adapting to local regulatory requirements [3]. With real-time insights into production, inventory, and compliance, manufacturers can respond quickly to market demands without compromising on safety or quality [2]. This combination of agility and compliance-readiness positions businesses to seize opportunities while navigating regulatory challenges with confidence.
Conclusion
ERP systems have become indispensable for manufacturers tackling the challenges of regulatory compliance. By centralizing data, automating audit trails, and enabling real-time monitoring, these systems deliver tangible results: a 40% reduction in audit preparation time and 52% fewer compliance-related errors [5].
For growing manufacturers, these advantages are game-changing. With regulations constantly evolving, relying on manual compliance processes is no longer practical. ERP systems solve this problem by offering automated reporting, role-based security, and built-in validation tools that adapt to new requirements - without the need for costly system overhauls.
Beyond avoiding penalties and compliance missteps, integrated ERP systems accelerate market entry by ensuring accurate and timely regulatory reporting. This allows businesses to capitalize on new opportunities while maintaining strict adherence to regulatory standards. The result? A powerful combination of efficiency and risk management that enables manufacturers to grow with confidence in highly regulated industries.
FAQs
How do ERP systems help minimize compliance errors?
ERP systems are a game-changer when it comes to cutting down compliance errors. By bringing together data from various departments into one centralized system, they ensure that all information stays consistent and up-to-date. This significantly reduces the risk of mistakes caused by outdated or conflicting data. On top of that, many ERP platforms come equipped with built-in tools like automated reporting and real-time monitoring, helping businesses stay on top of regulatory requirements without the hassle.
Features such as audit trails and standardized workflows add another layer of reliability. They make it easier to catch and resolve discrepancies early, before they escalate into bigger problems. This not only keeps errors in check but also saves valuable time and effort during audits or regulatory reviews.
How does centralized data help with regulatory compliance in manufacturing?
Centralized data acts as a single source of truth for your organization, keeping all essential information accurate, consistent, and readily available. By bringing data together into one system, businesses can gain real-time insights into their operations. This makes it easier to meet compliance requirements and reduces the chance of errors.
Having centralized data also streamlines the creation of audit trails and regulatory reports. These processes become faster and more automated, saving valuable time while ensuring reports meet the latest compliance standards. This helps manufacturers stay audit-ready and avoid costly penalties.
How do ERP systems use real-time monitoring to help with compliance?
ERP systems equipped with real-time monitoring keep a close eye on transactions and processes as they occur, flagging unusual activities or deviations from compliance standards right away. This kind of immediate detection allows businesses to tackle potential problems early, preventing them from growing into larger compliance issues.
With automated alerts and instant access to precise data, these systems minimize the chance of human error and ensure compliance standards are met consistently. The result? Meeting regulatory requirements becomes less of a hassle while also boosting overall operational efficiency.